- Location – Eastern Europe
- Population ~ 2,63mln
- 2018 GDP – 11.31 billion $
- Spoken language – Romanian
- Local currency – MDL
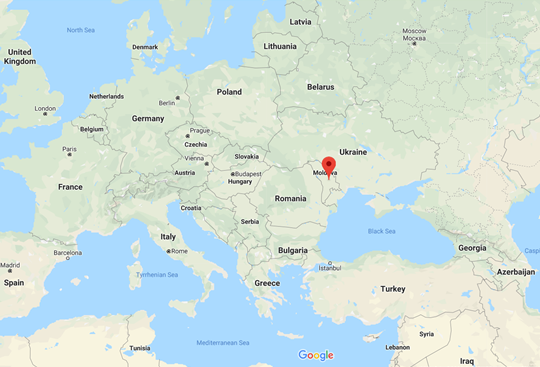
- Relatively small number of studies
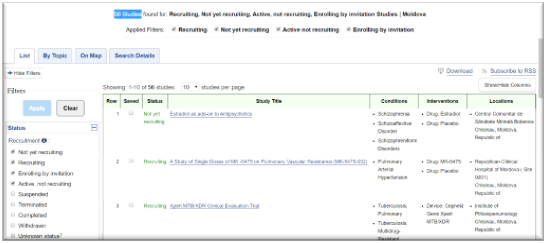
- CT approval dynamics in Moldova
- Source in Romanian – https://amdm.gov.md/sites/default/files/Despre%20Agentie/Raport%20pe%20activitate%20AMDM%20anul%202018.pdf
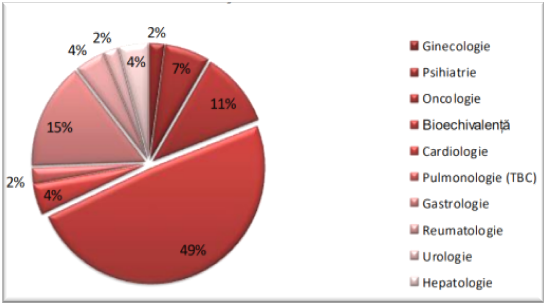
- Profile of approved CTs
- 49% – Bioequivalence CT
- 15% – Gastrology
- 11% – Oncology
- CT approval by phase in 2018
- Phase I: 9 CTs uPhase II: 3 CTs
- Phase III: 12 CTs uPhase IV: 1 CT
- Bioequivalence: 23 CTs
Peculiarities
- We are a team of experienced PM/CRAs (5+ years of experience in the field of CTs);
- We have established a network of doctors in all 18 biggest hospitals of the country, registered for the conduct of CT, covering all therapeutic areas;
- Centralized health care system with patient canalization in big hospitals – fast recruitment;
- Availability of physicians with experience in Clinical trials, good knowledge of English, PC knowledge (eCRF systems)
Secure your financial future through optimized portfolio management Volmar GrowthBeacon Handel